[2024 Flagship Brand] Ultra V, Bring “K-Slow Aging Beauty” to a Higher…
페이지 정보
작성자 ultrav 작성일24-08-06 16:10 조회1,162회관련링크
본문
[2024 Flagship Brand] Ultra V, Bring “K-Slow Aging Beauty” to a Higher Position
Biomedical beauty group Ultra V is considered as a representative player in K-beauty. Ultra V is introducing a variety of beauty products by holding the patented technology of ‘Idebenone’, a powerful skin antioxidant ingredient, and are receiving a lot of love not only in Korea but also in the overseas cosmetic medical market with ‘Ultracol’, a self-collagen regeneration skin booster. This photo is of Ultracol model Han Chae-ah. Image is provided by Ultra V
‘Health & Beauty (H&B)’, which emerged as the demand for ‘healthy beauty’ rapidly increased in the 2020s, has become a key keyword in the beauty industry. In the H&B field, it is of course ‘functional cosmetics’ that has achieved the most remarkable growth. As the interest in 'H&B' increases every year and the preference for so-called 'inner beauty' that 'fills the skin not only on the outside but also on the inside deeply' increases, the direction of 'functional cosmetics' is to diversify products that respond to the individually customized needs of consumers. began to focus on Among them, ‘Slow-aging’, which emerged as a development of ‘anti-aging’ with the motto ‘prevention of aging with natural beauty’, continues to grow every year as a new main trend in ‘K-beauty’. The current address of ‘slow aging’ pointed out by many beauty companies, including CJ Olive Young, is ‘expected growth.’ As of last year, sales of slow-aging products showed an increase of more than two times compared to the same period last year, and the overall category showed an average growth trend of 10% for about three years from 2020 to 2023.
While Korea's leading cosmetics conglomerates are launching product one after another, Ultra V is leading the domestic market as a representative of 'K-Beauty', especially 'K-Slow Aging Beauty', starting with its independently developed 'skin antioxidant' ingredients. Beyond this, it is the brand that is attracting the most attention in the global beauty market.
As a biomedical beauty group, Ultra V (CEO Kwon Han-jin) is considered a representative player in K-beauty. We are introducing a variety of beauty products by holding the patented technology of 'Idebenone', a powerful skin antioxidant ingredient, and are receiving a lot of love not only in Korea but also in the overseas beauty medical market with 'ULTRACOL', a self-collagen regeneration skin booster.
In particular, Ultracol laid the foundation for its entry into the Chinese mainland by completing licensing from Hainan Province's CFDA (now NMPA) last year, and is truly expecting meaningful performance this year.

▲ Ultra V KakaoTalk Gift 4 new gift packages
# ‘Slow Aging’ campaign key item ‘Idebenone Ampoule’ leading the US and European ampoule markets
Ultra V’s representative product, ‘Idebenone Ampoule’, has been popular since its launching in 2016 and is currently in its 3rd season and is considered the national ampoule. ‘Idebenone’ is recognized as the highest-grade antioxidant ingredient by the American Academy of Dermatology and boasts the most powerful anti-aging effect among existing ingredients. As a result, it proved to be effective in preventing skin aging as well as improving skin such as moisturizing, elasticity, wrinkles, whitening, and tone.
Meanwhile, recently, the concept of ‘slow aging’, which aims to slowly delay aging and pursue age-appropriate naturalness, focusing on the 2030 generation, is attracting attention, and the age group for products is expanding to the MZ group. As part of this, we opened a new store in ‘KakaoTalk Gift’ last February and exclusively introduced 4 types of gift packages based on Idebenone Ampoule.
Above all, in line with the characteristics of the gift platform, the quality of the gift has been raised with a luxurious package and a useful composition that anyone can use. In addition, we are targeting the consumer market through continuous communication with consumers by participating in the ‘Slow Aging’ campaign conducted by Olive Young.
In addition, Ultra V completed the European CPNP product registration based on the idebenone ampoule and entered European regions such as France and Greece. Thanks to the strength of K-beauty, it also entered the Costco online mall in the United States last year and is gradually expanding its sales area.
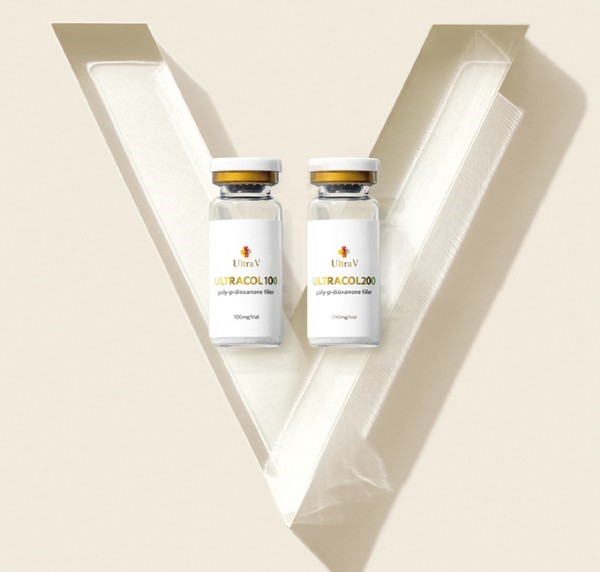
▲ 'UltraCol' is a product developed with Ultra V's proprietary technology. It is an injection that makes PDO (Polydioxanone), which is harmless to the human body, in the form of small microspheres and injects into the skin to induce self-collagen production. The photos show Ultracol 100 and 200 products.
# ‘Ultracol’, containing patented technology, obtains product approval certification from Hainan Province, China, and begins full-scale launch on Mainland China
'UltraCol' is a product developed with Ultra V's proprietary technology. It creates PDO (Polydioxanone), which is harmless to the human body, in the form of small microspheres and injects them into the skin to induce autologous collagen production.
PDO is an absorbable thread component mainly used for surgical suturing threads or thread lifting, and is safer because it naturally melts and disappears in human body. In addition, due to the nature of the ingredient, it naturally stimulates collagen production, which not only improves wrinkles and elasticity, but also improves problems such as sagging under the eyes, dark circles, nasolabial folds, facial pores, and skin texture.
Ultra V possesses the world's first PDO material self-collagen regeneration-inducing filler manufacturing and mass production technology, and Ultracol, launched based on this technology, has already introduced its products to markets around the world, including Asia, Europe, South America, and the Middle East, contributing to sales growth. This is a product that has contributed.
Moreover, making steady efforts to pioneer the Chinese market, in December of last year, Ultracol received product approval from Hainan Province in China and signed a sales-related business agreement (MOU) with leading Chinese companies. Subsequently, for full-scale launch in mainland China this year, biological safety evaluation is currently in progress, and clinical trials are planned to be submitted within 3 months.
In addition, Ultra V is currently in the process of registering Ultracol as an item to receive approval from ANVISA, Brazil's local Ministry of Food and Drug Safety, and is expected to receive product approval in June of this year. As soon as this process is completed, product sales in Brazil will begin.
An official from Ultra V said, “We expect that we will be able to achieve good results in the Chinese market by leveraging Ultracol’s effective safety and we plan to use this as a stepping stone to pursue listing on the KOSDAQ.”





