Ultra V Accelerates Global Expansion with UltraCol's Product Regi…
페이지 정보
작성자 ultrav 작성일24-11-04 18:12 조회308회관련링크
본문
Ultra V Accelerates Global Expansion with
UltraCol's Product Registration in Israel.
Bio-Medical Beauty Group Ultra V (CEO Han-jin Kwon) has further expanded its presence in the global market with the product registration approval of 'UltraCol' in Israel. This registration marks the 19th overseas authorization since its launch and is expected to further enhance its international credibility.
Ultra V has received market registration for UltraCol100 and UltraCol200, which were developed in-house using its unique patented technology, from the Ministry of Health of Israel.
Israel has a medical device market valued at approximately $3 billion annually, demonstrating continuous growth. Notably, active research and development in the life sciences and biotechnology sectors make it the largest medical device market in the Middle East, with significant growth potential for the future.
With this approval, Ultra V has established a foundation for the official start of sales in Israel and plans to quickly introduce its products to the market in collaboration with local distribution partners.
‘UltraCol' is produced by using a unique technology that involves breaking down the PDO (Polydioxanone) component at the molecular level and processing it into a microsphere form optimized for injection into the skin layers. It excels in collagen regeneration and volume formation, aiding in wrinkle improvement, elasticity enhancement and skin texture refinement.
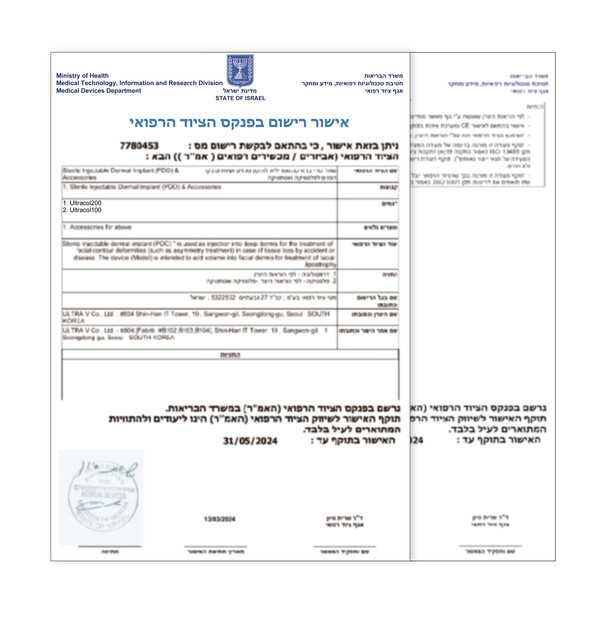
'UltraCol' has the advantage of being hydrolyzed within the body after the procedure, eliminating the need for separate removal. It has KFDA certification, European CE certification and CFDA certification from Hainan Province, China. Additionally, this product has received the IR52 Jang Young-sil Award and the Korea Patent Award for its unique patented technology, affirming its technological prowess and safety.
A representative from UltraV stated, "The product approval in Israel will be an important milestone for our global growth. We will actively establish tailored strategies for the local market in Israel to strengthen our global competitiveness and sustain our growth, while leading the global beauty medical industry.”
Meanwhile, Ultra V has achieved success in the Asian and European markets over the past few years, expanding its presence in the global aesthetic medical market. The company plans to continue seeking opportunities in new markets and pursue ongoing innovation.





