Ultra V, analysis of “the advantage in terms of competitiveness of non…
페이지 정보
작성자 ultrav 작성일24-12-21 16:08 조회1,410회관련링크
본문
Bio-medical beauty group UltraV (CEO Hanjin Kwon) declared that the official licensing in Hainan Province from China's CFDA (China Food and Drugs Administration; now NMPA- National Medical Products Administration) of the world’s first PDO MICROSPHERE ‘UltraCol’ has been completed, on Dec. 20.
Accordingly, Ultra V plans to use Hainan as a bridgehead to launch products and conduct aggressive marketing activities before supplying to mainland China in earnest. Attention is being paid to whether this will serve as an opportunity to improve Korea-China relations.
Above all, Ultra V is more meaningful as the first company in Korea to receive Hainan Province certification and approval from China's CFDA (now NMPA). In addition, there is no product that can replace ‘UltraCol’ in the Chinese aesthetic medical market, so it has a competitive advantage.
Invasion of mainland China from Hainan
Hainan is a representative tourist destination located in southern China. Since 2018, various pilot projects related to the construction of a free trade port have been implemented and an International medical tourism pilot zone has been created. Hainan is expected to secure a lot of demand as it falls under the medical tourism type among special medical zones.

A business agreement ceremony was held
at the Riviera Hotel Versailles Hall in Gangnam, where the 2023 UltraV &
The Master Clinic year-end party night event was held. (In order from left)
Sinopharm WAN QI, UltraV Kwon Kwon Jin-jin, TigerMed WAN PENGFEI, and Jang
Jin-hyuk, vice president of Warrather
According to Lee Suncheol, CEO of Woodway, the Chinese distributor, no new products have been launched in Hainan for about two years, and with the approval of UltraCol, the company expects to revolutionize the Chinese beauty market.
Expanding into the Chinese mainland market
China is one of the fastest growing medical aesthetics markets in the world and is estimated to be the largest aesthetic market after the US and Brazil. Since 2014, applications related to aesthetic medical procedures have emerged, contributing to the popularization of the procedures. Not only are more and more women pursuing anti-aging, but men are also planning aesthetic medical treatment, especially the younger generation of Generation MZ is emerging as the "mainstream".
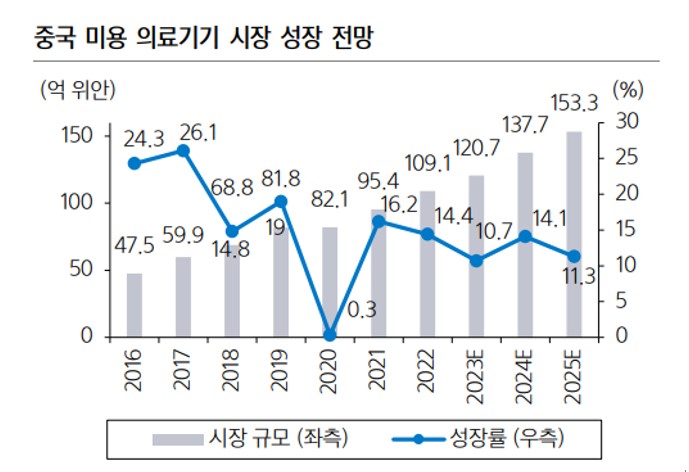
The Chinese filler market is expected to grow from 950 billion won (4.9 billion Chinese Yuan) in 2020 to nearly 3 trillion won (153 billion Chinese Yuan) in 2025. This is a huge opportunity considering that the domestic filler market is still in the 100 billion won range. As the market grows and policies become more normative, demand for high-value medical aesthetic devices and products is expected to continue to expand.
"In China, it is not easy to enter advanced medical technology and medical devices due to high barriers to entry, but Hainan is taking the initiative and leading the way," said an official from Ultra V. "We will use Hainan as an opportunity to publicize the technology of 'UltraCol' and gain experience and data on Chinese patients to enter the mainland market."
Ultracol is worldwide the first microparticle dermal filler based on polydioxanone (PDO)
By pioneering the Lifting absorbable threads market, UltraV has established ourselves as a leader in this industry. Through conferences and seminars in Korea and more than 60 countries around the world, UltraV has been delivering advanced and highly technical K-beauty medical aesthetic techniques to various countries.
In order to maximize the safety and effectiveness of skin
aesthetic procedures, the company has been making great efforts to develop
medical devices since its inception, and has developed world's first
microparticle dermal filler based on Polydoxanone (PDO), which is easily
degraded and safe, to stimulate the production of collagen.
'UltraCol' is a microsphere made of PDO that stimulates autologous collagen production to improve volume, lifting and elasticity, and is called the fourth generation of skin booster because it can also be used as a skin booster depending on the particle size. After receiving a Class 4 medical device license from the Ministry of Food and Drug Safety in Korea, the company has received CE certification and is the only company in Korea to receive the IR52 Received Jang Young Sil Award, and is actively engaged in various global activities.
As a product that has proven its quality reliability and safety, which can gain an edge in China's aesthetic medical market, the industry expects UltraCol to perform well in the mainland Chinese market, starting with Hainan, thanks to its effective safety.
Meanwhile, 'UltraCol' was recently selected as a 'Next Generation World Class Product' by the Ministry of Trade, Industry and Energy, officially recognizing its unique technology once again, and was awarded the 'Innovative Medical Device Industry Development Award' by the Ministry of Health and Welfare and the Seoul Mayor's Commendation Award as an outstanding company.





