NEWS & MEDIA
News
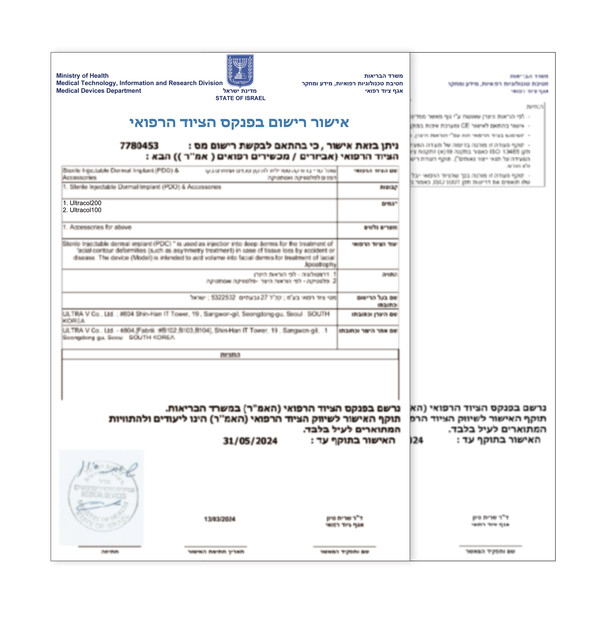
Ultra V Accelerates Global Expansion with UltraCol's Product Registration in Israel.
Bio-Medical Beauty Group Ultra V (CEO Han-jin Kwon) has further expanded its presence in the global market with the product registration approval of 'UltraCol' in Israel. This registration marks the 19th overseas authorization since its launch and is expected to further enhance its international credibility.
Ultra V has received market registration for UltraCol100 and UltraCol200, which were developed in-house using its unique patented technology, from the Ministry of Health of Israel.
Israel has a medical device market valued at approximately $3 billion annually, demonstrating continuous growth. Notably, active research and development in the life sciences and biotechnology sectors make it the largest medical device market in the Middle East, with significant growth potential for the future.
With this approval, Ultra V has established a foundation for the official start of sales in Israel and plans to quickly introduce its products to the market in collaboration with local distribution partners.
‘UltraCol' is produced by using a unique technology that involves breaking down the PDO (Polydioxanone) component at the molecular level and processing it into a microsphere form optimized for injection into the skin layers. It excels in collagen regeneration and volume formation, aiding in wrinkle improvement, elasticity enhancement and skin texture refinement.
A representative from UltraV stated, "The product approval in Israel will be an important milestone for our global growth. We will actively establish tailored strategies for the local market in Israel to strengthen our global competitiveness and sustain our growth, while leading the global beauty medical industry.”
Meanwhile, Ultra V has achieved success in the Asian and European markets over the past few years, expanding its presence in the global aesthetic medical market. The company plans to continue seeking opportunities in new markets and pursue ongoing innovation.



